

Revance RT001 is a topical treatment that is a neurotoxin or “neuromodulator”. This means that it decreases the muscle activity, similar to Botox Cosmetic, Dysport, and Xeomin. However, unlike the current FDA neuromodulators, Revance RT001 can penetrate through intact skin, whereas all the others require needle injections. Although the Botox injections are fairly quick and cause minimal discomfort, it is the belief by the company, Revance (RVNC), that there are many people that are avoiding treatment because of the needles. It has been reported that only 10% of the candidates for getting their crows feet treated are currently getting treated and that when offered a topical, the number of people getting treated will go up significantly. Unfortunately, the only area that has shown promise on the face in their studies has been the crows feet. The frown lines (glabella) treatment was not significantly improved with Revance RT001, and that’s most likely related to differences in the position of the muscles in this area (deep) when compared to the crows feet (superficial).
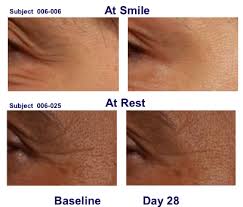
The treatment will be applied in the doctors office and will need to stay on the skin for approximately 20 minutes. This will NOT be a home remedy. Results might actually be better than the injections, for the crows feet, because the application will be more uniform to the area as opposed to the injections which are done in a sequential, interrupted manner.
Another use of Revance RT001 being researched is for excessive sweating, hyperhidrosis, in the underarm (axilla). Treating this area with the injectable neurotoxins (Botox, Dysport) requires about 30 punctures on each side. Clearly, Revance will be a huge step forward in comfort for patients being treated for this problem. A growing trend has been using Botox to treat genital/pubic area sweat. Needless to say, most people would prefer topicals in this area to several injections. It doesn’t seem to be effective in the hands or feet at this time because the thickness of the dermis prevents proper absorption of the drug through the skin. Research is also being done on its use for improving acne.
It looks like Revance RT001 will get FDA approval because of its great safety and efficacy in their current FDA trials. When will it get approved? Probably late 2014 or early 2015. This will be a welcome relief to all the needle-phobes with prominent crows feet.
Dr. Weiner is a Facial Plastic Surgeon who laid down his scalpel in 2005 and concentrates solely on noninvasive and minimally invasive Cosmetic Procedures. He is Johns Hopkins trained and Board Certified.
Website: http://www.theclinique.net
we’d love to meet you
Schedule a consultation
Are you ready to take the first step towards achieving your goals? Book Now or Contact Us first with any questions today!
Have a Question?




